David Ginsburg, M.D.


During his training in hematology and a year on the faculty at Harvard in hematology/oncology, David Ginsburg started to ask fundamental questions about how the blood clotting system works, especially how it works in humans. At Michigan, Ginsburg’s career has been distinguished in both clinical practice and basic research. As a physician, Ginsburg is board-certified in four specialties: hematology, oncology, internal medicine and clinical genetics. In his lab at the LSI, he investigates the fundamental biology and genetics of blood clotting.
Blood clotting has evolutionary roots are as far back as the advent of vertebrates. Invertebrates, by contrast, are just a pool of blood, and have no circulatory system. With evolutionary fine-tuning, the circulatory system’s clotting response—which involves the products of hundreds of genes—is carefully calibrated to be just right: too little clotting response, and even a minor injury would result in fatal bleeding, and too much clotting could produce blockages in critical blood vessels, leading to diseases such as strokes, heart attacks and lung embolisms.
As a postdoctoral fellow, Ginsburg cloned the gene for von Willebrand factor, one of the proteins that is critical to the cascade of reactions causing blood to clot, and which malfunctions in a relatively rare disorder known as von Willebrand disease. Since joining U-M in 1985, Ginsburg has identified the genes and mutations associated with many subtypes of von Willebrand disease and the specific gene mutations that bring them about. The work has scientific implications as well as impact on patients with the disease.
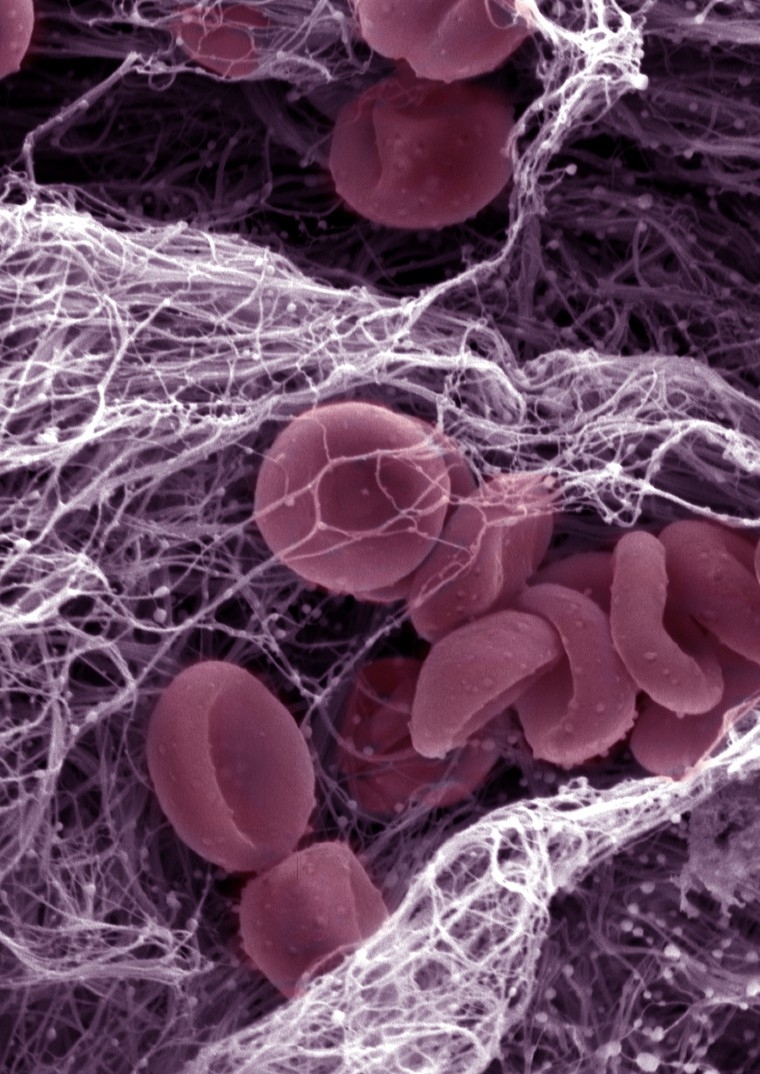
Contributions from the Ginsburg lab advanced the understanding of the common bleeding disorder von Willebrand disease (VWD), and identified modifier genes that may help determine the severity for VWD and related diseases.
The lab identified the VWF-processing metalloprotease ADAMTS13 as the cause of Thrombotic Thrombocytopenia Purpura (TTP) and the cause of combined deficiency of factors V and VIII as due to mutations in either of 2 genes (LMAN1 and MCFD2). LMAN1/MCFD2 form a specific cargo receptor for the transport of a select subset of proteins from the ER to the Golgi. Studies of mice genetically deficient in LMAN1 have provided insight into other potential cargos dependent on this novel pathway, as well as definitively establishing the endothelial cell as the biosynthetic source of plasma FVIII. The lab has characterized a number of key components of the plasminogen activation system, the mechanism by which blood clots are broken down.
Building on its discovery of the LMAN1/MCFD2 cargo receptor, the Ginsburg lab has recently extended its studies to more broadly explore the function of the COPII secretory pathway in mammalian cells, developing gene-targeted mice with deficiencies of the components of the inner COPII coat (SEC23A, SEC23B, and SEC24A-D) and discovering a surprisingly range of phenotypes in deficient animals.
This work led to the finding that the important cholesterol regulator, PCSK9, is specifically dependent on SEC24A for efficient exit from the ER. Analysis of SEC23 deficient mice has provided important insight into the relative functions of these two closely-related paralogs, as well as their role in the red blood cell genetic disorder, CDAII.