Research Highlight: New study reveals how some fungi initiate a crucial chemical reaction in nature’s lab
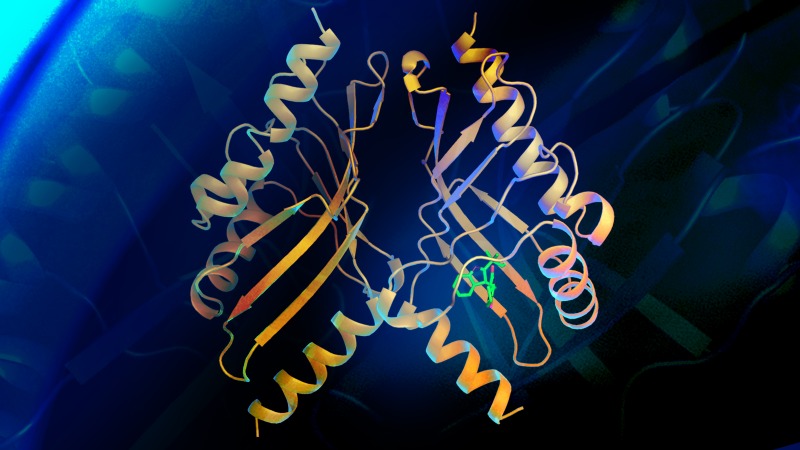
Structural overview of the pinacolase BvnE, with the substrate bound to the active site
An international team of researchers led by the University of Michigan Life Sciences Institute has decoded the complex process that fungi use to build an important class of bioactive compounds.
The compounds, called fungal indole alkaloids, have attracted considerable interest for their wide range of potentially beneficial properties — including anticancer and antiparasitic activities. Since their discovery more than 50 years ago, scientists have been trying to reconstruct their synthesis in the lab, with little success.
These new findings, published May 18 in Nature Catalysis, now offer a foundation from which chemists can begin to recreate the process to generate these compounds or new variants in the lab.
Scientists have long suspected that, to build these compounds, fungi use a reaction that is routine in organic chemistry labs but elusive in nature: the Diels-Alder cycloaddition reaction.
This group of researchers recently discovered a unique form of an enzyme called a Diels-Alderase that fungi employ to build one class of indole alkaloid compounds, including the commercial antiparasitic agent paraherquamide, thus answering a 50-year-old question about whether the Diels-Alder reaction could take place in nature to construct this metabolite.
In this most recent study, they found another method that fungi use to achieve this reaction spontaneously, without the use of a directing Diels Alderase biocatalyst.
“It turns out that, instead of using an enzyme to conduct the Diels-Alder reaction, the fungi produce a bunch of enzymes that work together to set up a structure in which the Diels-Alder cycloaddition occurs spontaneously — which is how organic chemists would set up the reaction in the lab,” explains Sean Newmister, Ph.D., a researcher at the LSI who contributed to the study.
To assemble the right compound, reactions must be directed to occur in precisely the right way. Even the tiniest change in the directions will result in a completely different product. In their previous study, the researchers found the Diels-Alderase directing the reaction. In this newest study, the directing took place just before the reaction occurred, through an enzyme called a pinacolase.
These findings corroborate a recent total synthesis study from another research group. In that work, the team devised a chemical synthesis route to building the version of the compound as it exists leading up the final step of its production, facilitating the spontaneous Diels-Adler reaction and generating the desired final product. The two complementary studies demonstrate how the fungi are able to initiate this key cycloaddition reaction without a Diels-Alderase, and underscore nature’s ability to evolve chemical reactions in surprising ways to produce complex, bioactive natural products.
“Nature doesn’t just choose one way to do things,” says David H. Sherman, Ph.D., a professor at the LSI and a lead author of the study. “And now we’re starting to show how many different ways nature has evolved to carry out this important reaction from different angles.”
Go to Article
“Fungal-derived brevianamide assembly by a stereoselective semipinacolase,” Nature Catalysis. DOI: 10.1038/s41929-020-0454-9.


