How does nature make it? Mapping cyanobacterial pathways in the search for new drugs
Research led by the University of Michigan Life Sciences Institute has mapped the complex chemistry involved in creating several types of bioactive compounds that are naturally produced inside bacteria.
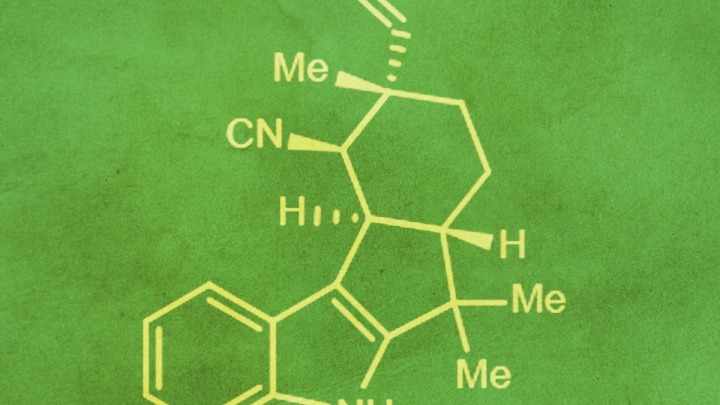
The compounds, known as hapalindole and fischerindole alkaloids, are members of a larger family of compounds — called indole alkaloids for their double-ring-shaped core structures — that have a long history of diverse uses including as antibacterial, antifungal and anticancer agents.
Not only does understanding the steps that the bacteria use to form these compounds pave the way for more easily producing them in the lab for medicinal purposes, it could allow scientists to selectively manipulate the process to create new variants, the researchers say.
The findings were published March 13 in Nature Chemical Biology.
“What nature has learned to produce very elegantly over millions of years of evolution can be difficult to reproduce in the laboratory, especially in large quantities,” says study lead author Shasha Li, a doctoral student in medicinal chemistry at the U-M College of Pharmacy and member of faculty member David Sherman’s lab at the LSI. “Because of their unique pharmacological properties and complex structures, we wanted to better understand the biochemical basis for the formation of these molecules. Our work has revised the prevailing hypotheses about how these compounds are made.”
By sequencing and analyzing the genome of Stigonematales cyanobacteria, the team was able to pinpoint several gene clusters that produce the proteins involved in various intermediary steps in the assembly of the complex, bioactive molecules.
Go to Article
Decoding cyclase-dependent assembly of hapalindole and fischerindole alkaloids, Nature Chemical Biology. DOI: 10.1038/nchembio.2327


