Ringing true: LSI scientists find solution to longstanding cell membrane puzzle
The biological membranes of cells can reshape themselves to form buds that send materials from the inside of the cell to far-flung places in the body. It happens in cells infected with HIV. It happens in cancer. And also in a host of other circumstances associated both with maintaining everyday health and with disease.
Scientists see enormous potential in developing the ability to inhibit or enhance budding by modulating the underlying cellular processes.
A team of researchers at the University of Michigan has now made an important step in that direction — they developed a clear picture of a critical mechanism in the budding process by obtaining the first three-dimensional snapshots of a protein complex at the heart of the action.
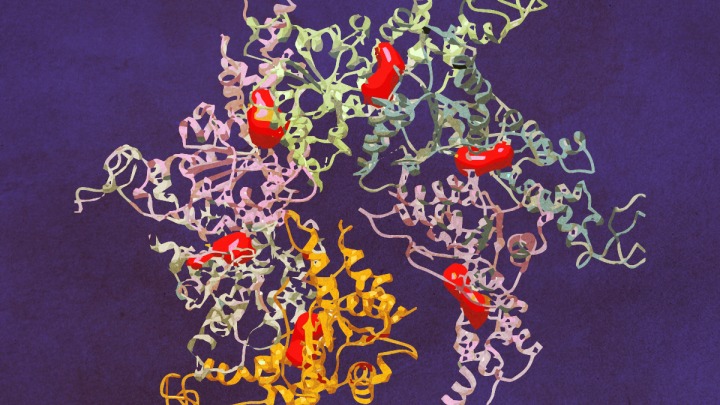
The structure of Vps4 (vacuolar protein sorting-associated protein 4) had previously been obtained through X-ray crystallography, however the new research — published April 14 in Science Advances — was the first to show how six copies of the protein actually come together to form an active molecular machine.
Defying scientists’ expectations, the resulting ring of six Vps4 molecules — called a hexamer — is asymmetrical, says co-senior author Zhaohui Xu, Ph.D. And the discovery of this asymmetry provides new insights into how the complex functions to dissemble another protein complex known as ESCRT-III (endosomal sorting complex required for transport III).

“There are many proteins that are involved in this process, but Vps4 is the only one that has enzymatic activity, so that presents an entry point for designing compounds to modulate this process,” says, Xu, a faculty member at the U-M Life Sciences Institute, where his lab is located, and an associate professor of biological chemistry in the Medical School.
Xu’s group had been working on understanding Vps4 for about a decade, but the new breakthrough came through teaming up with the lab of LSI faculty member Georgios Skiniotis, Ph.D. — in precisely the kind of interdisciplinary collaboration the LSI was designed to foster.
Skiniotis specializes in an imaging technique called cryo-electron microscopy. Cryo-EM involves freezing proteins in a thin layer of solution and then bouncing electrons off of them to reveal their shape. Because the frozen proteins are oriented every which way, computer software can later combine the thousands of individual snapshots into a 3-D picture at near-atomic resolution.
“By using cryo-EM we were able to capture the Vps4 hexamer in both its open and closed conformational states, showing for the first time what this biological machine really looks like and providing important information about how it functions,” says Skiniotis, the paper’s other senior author, who is also an associate professor of biological chemistry at the Medical School. “The hexamer is like a disc and likes to lay flat, so we had to overcome some technological challenges to get clear side-view images.”
The images obtained through cryo-EM, along with biochemistry experiments to test their hypotheses, allowed the researchers to propose a new model of how Vsp4 works: Only a single subunit of the hexamer is active at a time; and the action of the complex shifts which unit is active, so that subunits activate sequentially around the ring.
“So the cycle continues and pieces of the ESCRT-III protein are broken down one by one,” says Xu. “The beauty of this study is that by obtaining the cryo-EM structures we were able to propose a new mechanism that can explain how this complex actually disassembles the ESCRT-III complex.”
He adds, “This is somewhat of a skeletal model – there are many other regulators for this activity inside of our cells, so the next step is to build up from this model to continue to refine our understanding of how these processes work inside living organisms.”
Go to Article
Mechanism of Vps4 hexamer function revealed by cryo-EM, Science Advances. DOI: 10.1126/xciadv.1700325


