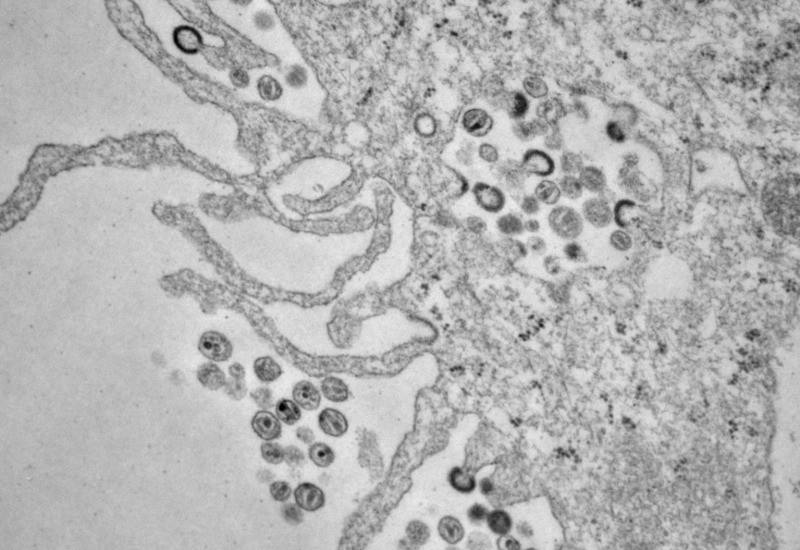
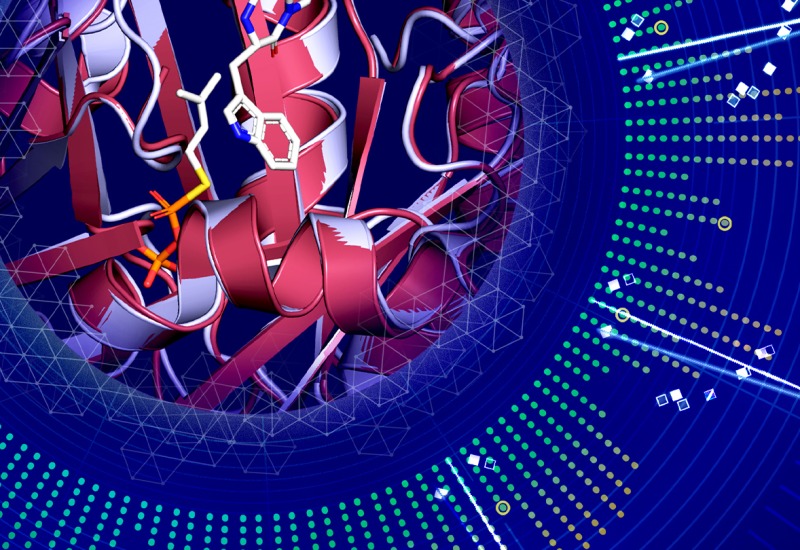
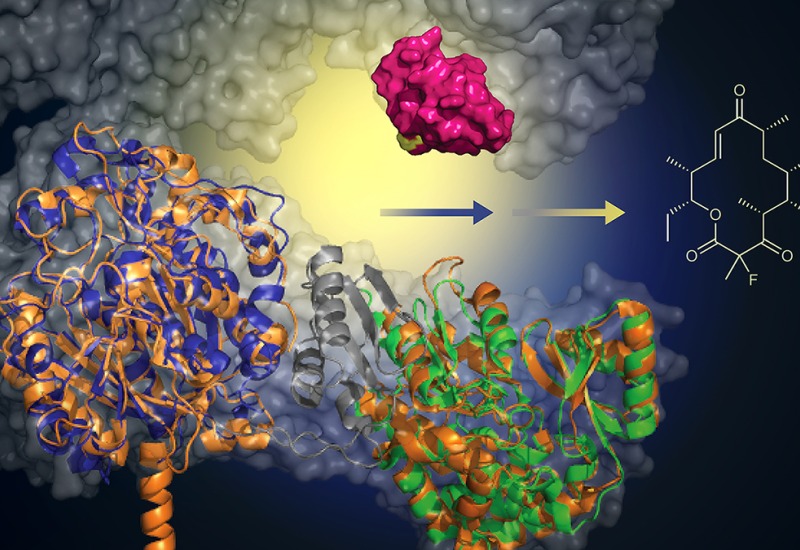
Composed of a dynamic, interdisciplinary team of scientists, the Sherman laboratory studies the biosynthesis of natural products from microbes that include cyanobacteria, actinomycetes, and myxobacteria. We are inspired by natural products from both terrestrial and marine organisms and seek to better understand their origins using a set of tools that includes molecular biology, genetics, biochemistry, structural biology, and bioorganic chemistry.
We harness the power of chemistry that has evolved inside of microorganisms to pioneer new antibiotics, anti-cancer drugs and other medicines.
Our Research
Image

David Sherman, Ph.D.
Research Professor, U-M Life Sciences Institute
Hans W. Vahlteich Professor of Medicinal Chemistry, U-M College of Pharmacy
Professor of Microbiology and Immunology, U-M Medical School
Professor of Chemistry, College of Literature, Science, and the Arts